Nicolas Moget, Jérémy Saiter, Fabien Toulgoat, Thierry Billard, Frédéric Leroux, Armen Panossian, Gilles Hanquet
Chem. Eur. J., 2025, 31, e02465
● Synthetic Route to Fluoroalkylselenylchalcogeno Molecules
Clément Delobel, Oz Barnier, Fabien Toulgoat, Thierry Billard
Eur. J. Org. Chem., 2025, 28, e202500561 📖
● A journey into trifluoromethylchalcogenation: some reagents from Lyon
Thierry Billard
C. R. Chim. 2024, 27, 217-226.

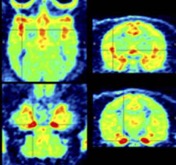
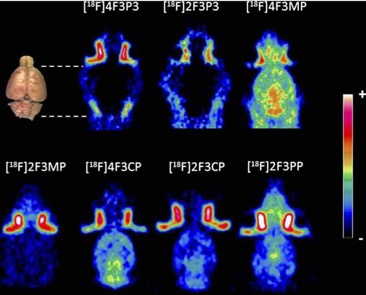
● Perspectives on obesity imaging: [18F]2FNQ1P a specific 5-HT6 brain PET radiotracer
P. Courault, S. Bouvard, C. Bouillot, R. Bolbos, W. Zeinyeh, T. Iecker, F. Liger, T. Billard, L. Zimmer, F. Chauveau, S. Lancelot
Int. J. Obes. 2025, 49, 133-139.

● CF3S-N bond formation under mild conditions. Easy access to trifluoromethanesulfenamides with a versatile reagent
Clément Delobel, Fabien Toulgoat, Thierry Billard
J. Fluorine Chem. 2024, 279, 110353.

● Phenylseleno trifluoromethoxylation of alkenes
C. Delobel, A. Panossian, G. Hanquet, F. R. Leroux, F. Toulgoat, T. Billard
Beilstein J.Org. Chem. 2024, 20, 2434-2441

● Deep Study of Thiocarbamoyl Fluorides: Synthesis and Properties
Clément Delobel, Emmanuel Chefdeville, Fabien Toulgoat, Thierry Billard
Adv. Synth. Catal. 2024, 366, 3474-3480

● WAY-208466, a 5-HT6 receptor agonist, increases food motivation in primates: A behavioural and PET imaging study opening perspectives in eating disorders
M. Pitoy, J. Maulavé, L. Gauthier, J. Debatisse, N. Costes, I. Mérida, T. Billard, K. Portier, S. Lancelot, B. Galusca, L. Zimmer, L. Tremblay
Neuroscience Applied 2024, 3, 104086

● Trifluoromethoxylation of Arynes Using 2,4-Dinitro-1-(trifluoromethoxybenzene) as Trifluoromethoxide Anion Source
L. Wisson, G. Hanquet, F. Toulgoat, T. Billard, A. Panossian, F. R. Leroux
Eur. J. Org. Chem. 2024, 27, e202400388 📖

● Unlocking the Power of Acyl Fluorides: A Comprehensive Guide to Synthesis and Properties
C. Bonnefoy, A. Gallego, C. Delobel, B. Raynal, M. Decourt, E. Chefdeville, G. Hanquet, A. Panossian, F. R. Leroux, F. Toulgoat, T. Billard
Eur. J. Org. Chem. 2024, 27, e202400142 📖
● SB-258585 reduces food motivation while blocking 5-HT6 receptors in the non-human primate striatum
M. Pitoy, L. Gauthier, J. Debatisse, J. Maulavé, E. Météreau, M. Beaudoin, K. Portier, V. Sgambato, T. Billard, L. Zimmer, S. Lancelot, L. Tremblay
Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 131, 110970
● Difluoromethoxylated Ketones as Building Blocks for the Synthesis of Challenging OCF2H-Bearing N-Heterocycles
A. Loison, G. Hanquet, F. Toulgoat, T. Billard, A. Panossian, F. R. Leroux
Eur. J. Org. Chem. 2023, 26, e202300695 📖

● Comprehensive Study and Development of a Metal-Free and Mild Nucleophilic Trifluoromethoxylation
C. Bonnefoy, A. Panossian, G. Hanquet, F. R. Leroux, F. Toulgoat, T. Billard
Chem. Eur. J. 2023, 29, e202301513 📖

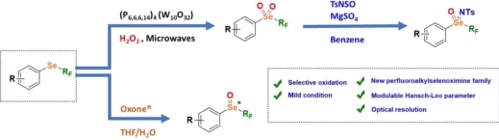
● Perfluoroalkyl Selenoxides, Selenones and Selenoximines: General Preparation and Properties
A. de Zordo-Banliat, K. Grollier, N. Vanthuyne, S. Floquet, Th. Billard, G. Dagousset, B. Pégot, E. Magnier
Angew. Chem. Int. Ed. 2023, 62, e202300951
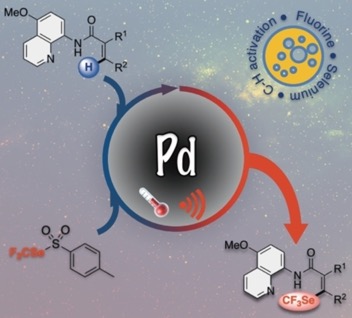
● Vinylic Trifluoromethylselenolation via Pd-Catalyzed C−H Activation
A. de Zordo-Banliat, K. Grollier, J. Vigier, E. Jeanneau, G. Dagousset, B. Pegot, E. Magnier, Th. Billard
Chem. Eur. J. 2022, 28, e202202299 📖
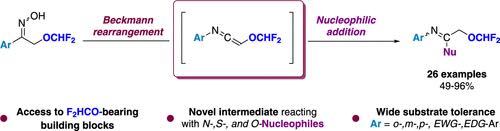
● Ketenimines as Intermediates To Access Difluoromethoxylated Scaffolds
A. Loison, G. Hanquet, F. Toulgoat, T. Billard, A. Panossian, F. R. Leroux
Org. Lett. 2022, 24, 8316-8321
● Study of Carbamoyl Fluoride: Synthesis, Properties and Applications
C. Bonnefoy, E. Chefdeville, C. Tourvieille, A. Panossian, G. Hanquet, F. Leroux, F. Toulgoat, Th. Billard
Chem. Eur. J. 2022, 28, e202201589 📖
Référencé dans les actualités de l'INC CNRS.
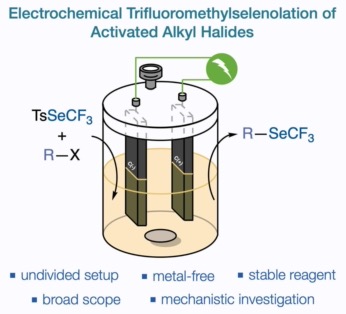
● Electrochemical Trifluoromethylselenolation of Activated Alkyl Halides
K. Grollier, C. Ghiazza, A. Tlili, T. Billard, M. Médebielle, J. C. Vantourout
Eur. J. Org. Chem. 2022, e202200123
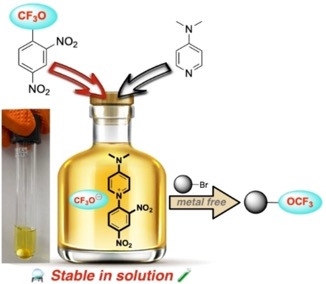
● Study of a Stable “Trifluoromethoxide Anion Solution” Arising from 2,4-Dinitro-Trifluoromethoxybenzene
C. Bonnefoy, E. Chefdeville, A. Panossian, G. Hanquet, F. R. Leroux, F. Toulgoat, T. Billard
Chem. Eur. J. 2021, 27, 15986-15991 📖
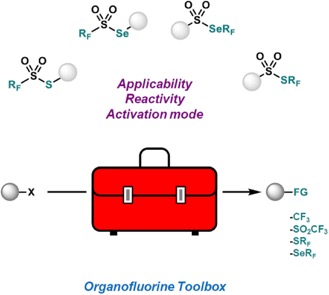
● Synthesis, Reactivity and Activation Modes of Fluoroalkyl Thiosulfonates and Selenosulfonates
C. Ghiazza, T. Billard
Eur. J. Org. Chem. 2021, 5571-5584
● Solvent free nucleophilic selenocyanation with [bmim][SeCN]. Direct access to perfluoroalkylselenide compounds
A. De-Zordo Banliat, K. Grollier, A. Damond, T. Billard, G. Dagousset, E. Magnier, B. Pégot
Tetrahedron 2021, 101, 132507

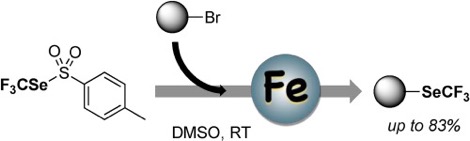
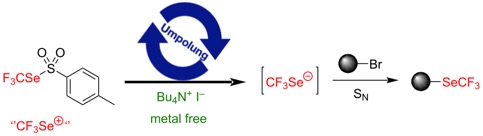
● Fe-mediated nucleophilic trifluoromethylselenolation of activated alkyl bromides via umpolung reactivity of trifluoromethyl tolueneselenosulfinate
K. Grollier, E. Chefdeville, A. De Zordo-Banliat, B. Pegot, G. Dagousset, E. Magnier, T. Billard
Tetrahedron 2021, 100, 132498
● Recent synthetic methods towards the –OCHF2 moiety
A. Loison, F. Toulgoat, T. Billard, G. Hanquet, A. Panossian, F. R. Leroux
Tetrahedron 2021, 99, 132458

● Aromatic Trifluoromethylselenolation via Pd-catalyzed C−H functionalization
K. Grollier, E. Chefdeville, E. Jeanneau, T. Billard
Chem. Eur. J. 2021, 27, 12910-12916 📖
● Different Alterations of Agonist and Antagonist Binding to 5-HT 1A Receptor in a Rat Model of Parkinson’s Disease and Levodopa-Induced Dyskinesia: A MicroPET Study
B. Vidal, E. Levigoureux, S. Chaib, C. Bouillot, T. Billard, A. Newman-Tancredi, L. Zimmer
J. Parkinsons Dis. 2021, 11, 1257-1269.
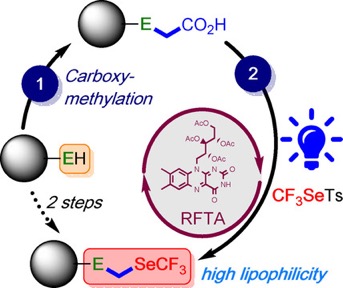
● (Trifluoromethylselenyl)methylchalcogenyl as Emerging Fluorinated Groups: Synthesis under Photoredox Catalysis and Determination of the Lipophilicity
K. Grollier, A. De Zordo-Banliat, F. Bourdreux, B. Pegot, G. Dagousset, E. Magnier, T. Billard
Chem. Eur. J. 2021, 27, 6028-6033. 📖
● [18F]F13640, a 5-HT1A Receptor Radiopharmaceutical Sensitive to Brain Serotonin Fluctuations
M. Colom, B. Vidal, S. Fieux, J. Redoute, N. Costes, F. Lavenne, I. Mérida, Z. Irace, T. Iecker, C. Bouillot, T. Billard, A. Newman-Tancredi, L. Zimmer
Front. Neurosci. 2021, 15, doi:10.3389/fnins.2021.622423.
● Chapitre de Livre / Book Chapter :
Chemistry of OCF3, SCF3, and SeCF3 Functional Groups
F. Toulgoat, F. Liger, T. Billard
in Organofluorine Chemistry: Synthesis, Modeling, and Applications. (Eds.: K. Szabó, N. Selander), Wiley‐VCH Verlag, Weinheim, Germany, 2021, pp. 49-97.

● Metal-free nucleophilic trifluoromethylselenolation via an iodide-mediated umpolung reactivity of trifluoromethylselenotoluenesulfonate
K. Grollier, A. Taponard, A. De Zordo-Banliat, E. Magnier, T. Billard
Beilstein J. Org. Chem. 2020, 16, 3032-3037 (Special issue, on invitation).
● Synthetic Approaches to Fluoroalkyltelluryl-Substituted Compounds
K. Grollier, A. Taponard, T. Billard
Eur. J. Org. Chem. 2020, 6943-6954. 📖

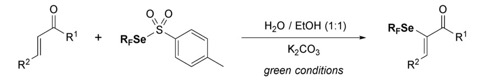
● Environmentally Compatible Access to α-Trifluoromethylseleno-Enones
K. Grollier, A. Taponard, C. Ghiazza, E. Magnier, T. Billard
Helv. Chim. Acta 2020, 103, e2000185 (Special issue dedicated to Antonio Togni ; on invitation). 📖
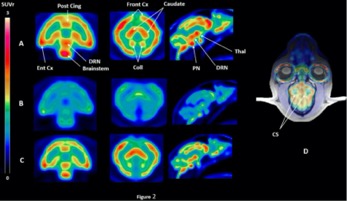
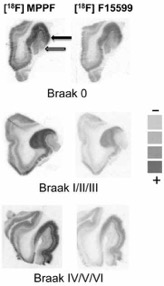
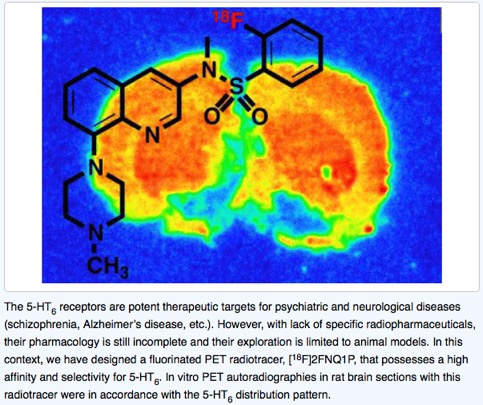
● Change in Expression of 5-HT 6 Receptor at Different Stages of Alzheimer’s Disease: A Postmortem Study with the PET Radiopharmaceutical [ 18F]2FNQ1P
P. Courault, S. Emery, S. Bouvard, F. Liger, F. Chauveau, D. Meyronet, A. Fourier, T. Billard, L. Zimmer, S. Lancelot
J. Alzheimer's Dis. 2020, 75, 1329-1338.
● Chapitre de Livre / Book Chapter :
When Fluorine Meets Selenium
T. Billard, F. Toulgoat
in Emerging Fluorinated Motifs. (Eds.: D. Cahard, J. A. Ma), Wiley‐VCH Verlag, Weinheim, Germany, 2020, Vol. 2, pp. 691-721.


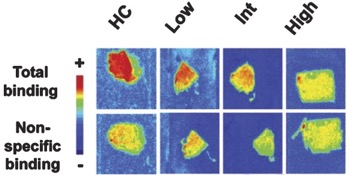
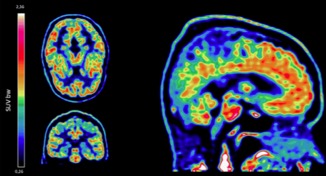
● Preclinical validation of [18F]2FNQ1P as a specific PET radiotracer of 5-HT6 receptors in rat, pig, non-human primate and human brain tissue
S. Emery, S. Fieux, B. Vidal, P. Courault, S. Bouvard, C. Tourvieille, T. Iecker, T. Billard, L. Zimmer, S. Lancelot
Nucl. Med. Biol. 2020, 82-83, 57-63.
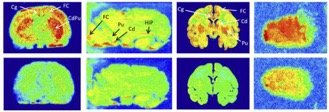
● 18F-F13640 PET imaging of functional receptors in humans
M. Colom, N. Costes, J. Redouté, F. Dailler, F. Gobert, D. Le Bars, T. Billard, A. Newman-Tancredi, L. Zimmer
Eur. J. Nucl. Med. Mol. Imag. 2020, 47, 220-221.
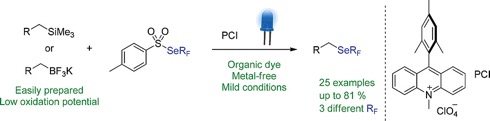
● Fluoroalkylselenolation of Alkyl Silanes/Trifluoroborates under Metal-Free Visible-Light Photoredox Catalysis
C. Ghiazza, L. Khrouz, T. Billard, C. Monnereau, A. Tlili
Eur. J. Org. Chem. 2020, 1559-1566.
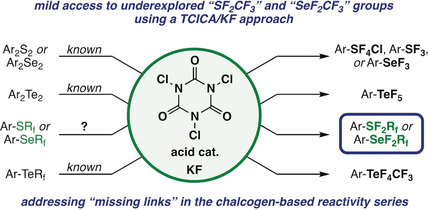
● Difluoro(aryl)(perfluoroalkyl)-λ4-sulfanes and Selanes: Missing Links of Trichloroisocyanuric Acid/Potassium Fluoride Chemistry
F. Brüning, C. R. Pitts, J. Kalim, D. Bornemann, C. Ghiazza, J. de Montmollin, N. Trapp, T. Billard, A. Togni
Angew. Chem. Int. Ed. 2019, 58, 18937-18941.
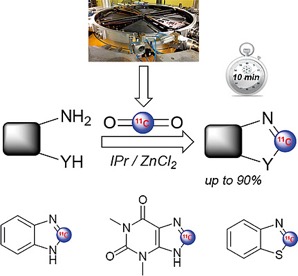
● 11C-Labeling: Intracyclic Incorporation of Carbon-11 into Heterocycles
F. Liger, F. Cadarossanesaib, T. Iecker, C. Tourvieille, D. Le Bars, T. Billard
Eur. J. Org. Chem. 2019, 6968-6972. 📖
Highlighted on the ChemistryViews website
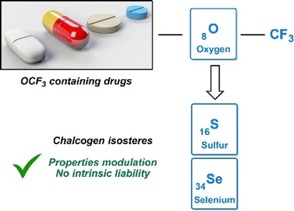
● Chalcogen OCF3 Isosteres Modulate Drug Properties without Introducing Inherent Liabilities
C. Ghiazza, T. Billard, C. Dickson, A. Tlili, C. M. Gampe
ChemMedChem 2019, 14, 1586-1589.
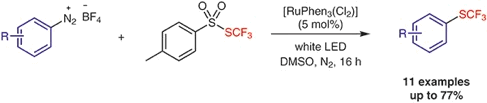
● Visible-Light-Mediated Synthesis of Trifluoromethylthiolated Arenes
C. Ghiazza, C. Monnereau, L. Khrouz, T. Billard, A. Tlili
Synthesis 2019, 51, 2865-2870.
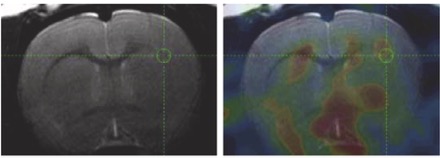
● Evaluation of Myelin Radiotracers in the Lysolecithin Rat Model of Focal Demyelination: Beware of Pitfalls!
M. Zhang, G. Hugon, C. Bouillot, R. Bolbos, J.-B. Langlois, T. Billard, F. Bonnefoi, B. Li, L. Zimmer, F. Chauveau
Contrast Media Mol. Imaging 2019, Article ID 9294586, doi:10.1155/2019/9294586.
● Umpolung Reactivity of Fluoroalkylselenotoluenesulfonates: Towards a Versatile Reagent
C. Ghiazza, A. Kataria, A. Tlili, F. Toulgoat, T. Billard
Asian J. Org. Chem. 2019, 8, 675-678 (Special Issue: Organofluorine Chemistry ; on invitation).
Highlighted on the ChemistryViews website

● Merging Visible-Light Catalysis for the Direct Late-Stage Group-16–Trifluoromethyl Bond Formation
C. Ghiazza, T. Billard, A. Tlili
Chem. Eur. J. 2019, 25, 6482-6495.
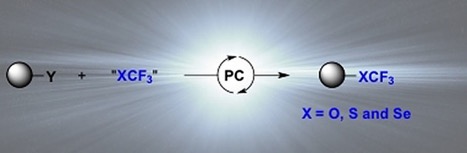
● New Avenues in Radical Trifluoromethylselenylation with Trifluoromethyl Tolueneselenosulfonate
C. Ghiazza, C. Monnereau, L. Khrouz, M. Médebielle, T. Billard, A. Tlili
Synlett 2019, 30, 777-782.
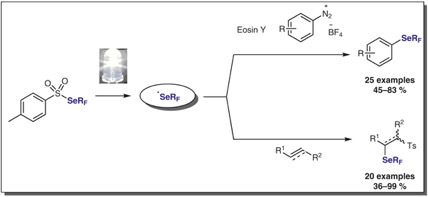
● Evaluation of [18F]2FP3 in pigs and non-human primates
H. D. Hansen, C. C. Constantinescu, O. Barret, M. M. Herth, J. H. Magnussen, S. Lehel, A. Dyssegaard, J. Colomb, T. Billard, L. Zimmer, G. Tamagnan, G. M. Knudsen
J. Labelled Compd. Radiopharm. 2019, 62, 34-42.
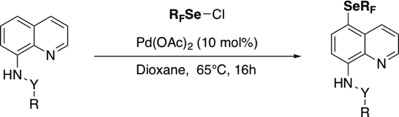
● Regioselective remote CH fluoroalkylselenolation of 8-aminoquinolines
C. Ghiazza, M. Ndiaye, A. Hamdi, A. Tlili, T. Billard
Tetrahedron 2018, 74, 6521-6526.
● Chapitre de Livre / Book Chapter :
Serotonin receptor imaging by 18F-PET.
T. Billard, F. Liger, M. Verdurand
in Fluorine in Life Sciences: Pharmaceuticals, Medicinal Diagnostics, and Agrochemicals. (Eds.: G. Haufe, F. Leroux), Elsevier Science, London, United Kingdom, 2018, pp. 459-518.


● Visible-light promoted fluoroalkylselenolation: toward the reactivity of unsaturated compounds
C. Ghiazza, L. Khrouz, C. Monnereau, T. Billard, A. Tlili
Chem. Commun. 2018, 54, 9909-9912.
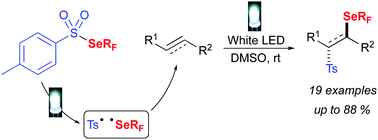
● Visible-Light-Mediated Metal-Free Synthesis of Trifluoromethylselenolated Arenes
C. Ghiazza, V. Debrauwer, C. Monnereau, L. Khrouz, M. Médebielle, T. Billard, A. Tlili
Angew. Chem. Int. Ed. 2018, 57, 11781-11785.
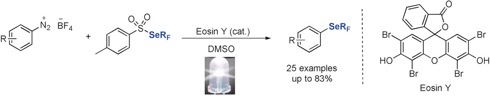
● In Silico, in Vitro, and in Vivo Evaluation of New Candidates for α-Synuclein PET Imaging
M. Verdurand, E. Levigoureux, W. Zeinyeh, L. Berthier, M. Mendjel-Herda, F. Cadarossanesaib, C. Bouillot, T. Iecker, R. Terreux, S. Lancelot, F. Chauveau, T. Billard, L. Zimmer
Mol. Pharm. 2018, 15, 3153-3166.
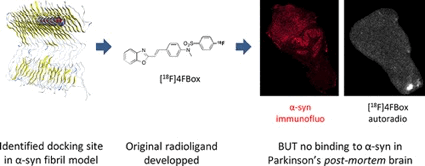
● Direct α-C–H Trifluoromethylselenolation of Carbonyl Compounds
C. Ghiazza, A. Tlili, T. Billard
Eur. J. Org. Chem. 2018, 3680-3683.
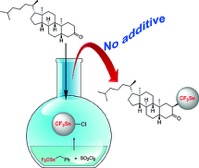
● 18F-F13640 preclinical evaluation in rodent, cat and primate as a 5-HT1A receptor agonist for PET neuroimaging
B. Vidal, S. Fieux, M. Colom, T. Billard, C. Bouillot, O. Barret, C. Constantinescu, G. Tamagnan, A. Newman-Tancredi, L. Zimmer
Brain Struct. Funct. 2018, 223, 2973-2988.
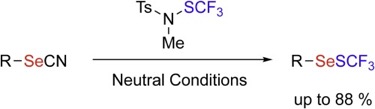
● Nucleophilic trifluoromethylthiolation of organoselenocyanates with trifluoromethanesulfenamide reagent: Access to CF3SSe-containing compounds
Q. Glenadel, C. Ayad, M.-A. D’Elia, T. Billard, F. Toulgoat
J. Fluorine Chem. 2018, 210, 112-116.
● Synthetic Approaches to Trifluoromethylselenolated Compounds
A. Tlili, E. Ismalaj, Q. Glenadel, C. Ghiazza, T. Billard
Chem. Eur. J. 2018, 24, 3659-3670.
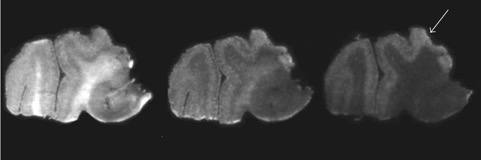
● Amyloid-Beta Radiotracer [18F]BF-227 Does Not Bind to Cytoplasmic Glial Inclusions of Postmortem Multiple System Atrophy Brain Tissue
M. Verdurand, E. Levigoureux, S. Lancelot, W. Zeinyeh, T. Billard, I. Quadrio, A. Perret-Liaudet, L. Zimmer, F. Chauveau
Contrast Media Mol. Imaging 2018, Article ID 9165458.
● Fluorous l-Carbidopa Precursors: Highly Enantioselective Synthesis and Computational Prediction of Bioactivity
A. Granados, A. d. Olmo, F. Peccati, T. Billard, M. Sodupe, A. Vallribera
J. Org. Chem. 2018, 83, 303-313.
● A Metal-Free Route to Heterocyclic Trifluoromethyl- and Fluoroalkylselenolated Molecules
Q. Glenadel, E. Ismalaj, T. Billard
Org. Lett. 2018, 20, 56-59.
● Exploring the Reactivity of Trifluoromethyl Tolueneselenosulfonate with Alkynes under Copper Catalysis
C. Ghiazza, V. Debrauwer, T. Billard, A. Tlili
Chem. Eur. J. 2018, 24, 97-100.
Highlighted on the ChemistryViews website

● Electrophilic trifluoromethylselenolation of terminal alkynes with Se-(trifluoromethyl) 4-methylbenzenesulfonoselenoate
C. Ghiazza, A. Tlili, T. Billard
Beilstein J. Org. Chem. 2017, 13, 2626-2630 (Special issue, on invitation).
● An easier and quicker access to (benzenesulfonyl)difluoromethanesulfenamide reagent
E. Ismalaj, T. Billard
J. Fluorine Chem. 2017, 203, 215-217 (Special issue, on invitation).
● Copper-Catalyzed Direct Trifluoro- and Perfluoroalkylselenolations of Boronic Acids with a Shelf-Stable Family of Reagents
Q. Glenadel, C. Ghiazza, A. Tlili, T. Billard
Adv. Synth. Catal. 2017, 359, 3414-3420.

● [11C]PF-3274167 as a PET radiotracer of oxytocin receptors: Radiosynthesis and evaluation in rat brain
B. Vidal, I. A. Karpenko, F. Liger, S. Fieux, C. Bouillot, T. Billard, M. Hibert, L. Zimmer
Nucl. Med. Biol. 2017, 55, 1-6.
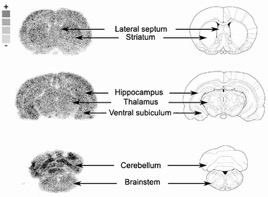
● Fluor et Santé (Fluorine and health)
T. Billard, E. Magnier
Actualité Chimique 2017, 421, 31-34 (Special issue, on invitation).

● Trifluoromethyl- and Fluoroalkylselenolations of Alkynyl Copper(I) Compounds
C. Ghiazza, T. Billard, A. Tlili
Chem. Eur. J. 2017, 23, 10013-10016.
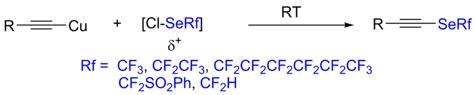
● Characterization and Reliability of [18F]2FNQ1P in Cynomolgus Monkeys as a PET Radiotracer for Serotonin 5-HT6 Receptors
V. Sgambato-Faure, T. Billard, E. Météreau, S. Duperrier, S. Fieux, N. Costes, L. Tremblay, L. Zimmer
Front. Pharmacol. 2017, 8, doi:10.3389/fphar.2017.00471.
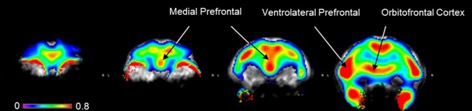
● Trifluoromethylselenolation and Fluoroalkylselenolation of Alkenes by Electrophilic Addition
C. Ghiazza, Q. Glenadel, A. Tlili, T. Billard
Eur. J. Org. Chem. 2017, 3812-3814.
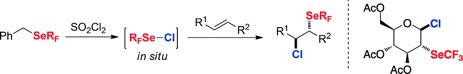
● Electrophilic Trifluoromethylselenolation of Boronic Acids
C. Ghiazza, A. Tlili, T. Billard
Molecules 2017, 22, 833-841 (Special issue, on invitation).
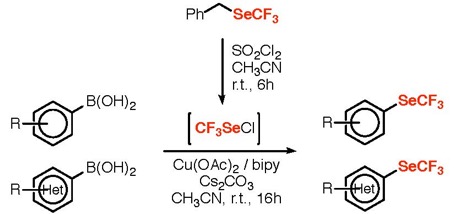
● Easy Access to SCF2-Containing Molecules through a Versatile Reagent
E. Ismalaj, Q. Glenadel, T. Billard
Eur. J. Org. Chem. 2017, 1911-1914.
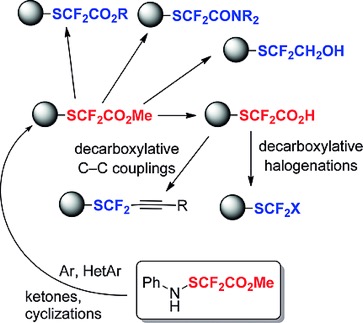
● Generation of the SCF3 Radical by Photoredox Catalysis: Intra- and Intermolecular Carbotrifluoromethylthiolation of Alkenes
G. Dagousset, C. Simon, E. Anselmi, B. Tuccio, T. Billard, E. Magnier
Chem. Eur. J. 2017, 23, 4282-4286.

● Chapitre de Livre / Book Chapter :
Towards CF3S Group: From Trifluoromethylation of Sulfides to Direct Trifluoromethylthiolation.
F. Toulgoat, T. Billard
in Modern Synthesis Processes and Reactivity of Fluorinated Compounds: Progress in Fluorine Science (Eds.: H. Groult, F. Leroux, A. Tressaud), Elsevier Science, London, United Kingdom, 2017, pp. 141-179.


● Electrophilic Trifluoromethyl- and Fluoroalkylselenolation of Organometallic Reagents
Q. Glenadel, E. Ismalaj, T. Billard
Eur. J. Org. Chem. 2017, 530-533.
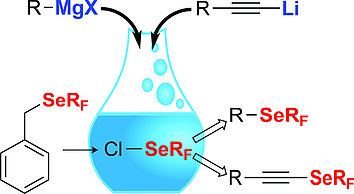
● Superacid-Catalyzed Trifluoromethylthiolation of Aromatic Amines
L. J. C. Bonazaba Milandou, H. Carreyre, S. Alazet, G. Greco, A. Martin-Mingot, C. Nkounkou Loumpangou, J.-M. Ouamba, F. Bouazza, T. Billard, S. Thibaudeau
Angew. Chem. Int. Ed. 2017, 56, 169-172.
● Methanesulfonic Acid, 1,1,1-Trifluoro-, Trifluoromethyl Ester
T. Billard.
Encyclopedia of Reagents for Organic Synthesis, 2016.
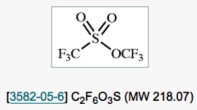
● Benzyltrifluoromethyl (or Fluoroalkyl) Selenide: Reagent for Electrophilic Trifluoromethyl (or Fluoroalkyl) Selenolation
Q. Glenadel, E. Ismalaj, T. Billard
J. Org. Chem. 2016, 81, 8268-8275.
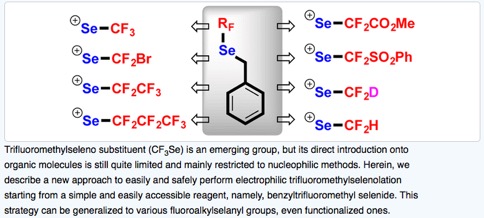
● Synthetic Approaches to Trifluoromethoxy-Substituted Compounds
A. Tlili, F. Toulgoat, T. Billard
Angew. Chem. Int. Ed. 2016, 55, 11726-11735.
● Copper-Catalyzed Perfluoroalkylthiolation of Alkynes with Perfluoroalkanesulfenamides
A. Tlili, S. Alazet, Q. Glenadel, T. Billard
Chem. Eur. J. 2016, 22, 10230-10234.
Highlighted on the ChemistryViews website
● Agonist and antagonist bind differently to 5-HT1A receptors during Alzheimer’s disease: A post-mortem study with PET radiopharmaceuticals
B. Vidal, J. Sebti, M. Verdurand, S. Fieux, T. Billard, N. Streichenberger, C. Troakes, A. Newman-Tancredi, L. Zimmer
Neuropharmacology 2016, 109, 88-95.
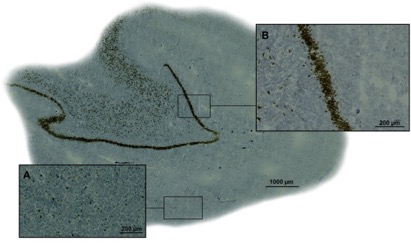
● Trifluoromethanesulfenamides: new reagents for direct S-CF3 bond formation
T. Billard
Chim. Oggi - Chem. Today 2016, 34, 18-21 (on invitation).

● Multigram Scale Syntheses of First and Second Generation of Trifluoromethanesulfenamide Reagents
Q. Glenadel, S. Alazet, F. Baert, T. Billard
Org. Process Res. Dev. 2016, 20, 960-964.

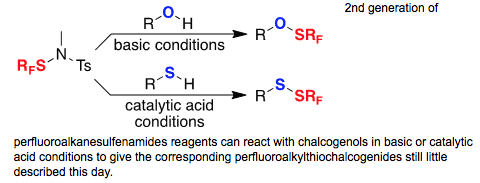
● Direct Perfluoroalkylthiolation of Few Chalcogenols
Q. Glenadel, T. Billard
Chin. J. Chem . 2016, 34, 455-458 (Special issue, on invitation).
● Metal-Free Direct Dehydroxytrifluoromethylthiolation of Alcohols via the Umpolung Reactivity of Trifluoromethanesulfenamides
Q. Glenadel, A. Tlili, T. Billard
Eur. J. Org. Chem. 2016, 1955-1957.

● Direct Electrophilic (Benzenesulfonyl)Difluoromethylthiolation with a Shelf-Stable Reagent
E. Ismalaj, D. Le Bars, T. Billard
Angew. Chem. Int. Ed. 2016, 55, 4790-4793.

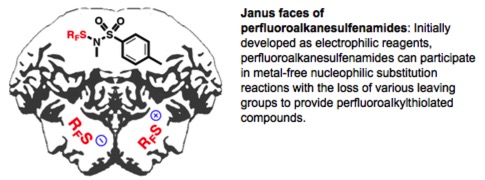
● Metal-Free Direct Nucleophilic Perfluoroalkylthiolation with Perfluoroalkanesulfenamides
Q. Glenadel, M. Bordy, S. Alazet, A. Tlili, T. Billard
Asian J. Org. Chem. 2016, 5, 428-433.
● First and second generation of trifluoromethanesulfenamide reagent: A trifluoromethylthiolating comparison
Q. Glenadel, S. Alazet, T. Billard
J. Fluorine Chem. 2015, 179, 89-95 (Special issue, on invitation).

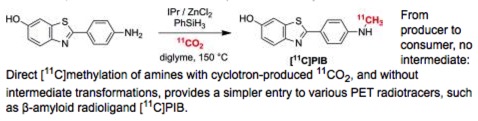
● Direct [11C]Methylation of Amines from [11C]CO2 for the Synthesis of PET Radiotracers
F. Liger, T. Eijsbouts, F. Cadarossanesaib, C. Tourvieille, D. Le Bars, T. Billard
Eur. J. Org. Chem. 2015, 6434-6438.
● Mild and Soft Catalyzed Trifluoromethylthiolation of Boronic Acids: The Crucial Role of Water
Q. Glenadel, S. Alazet, A. Tlili, T. Billard
Chem. Eur. J. 2015, 21, 14694-14698.
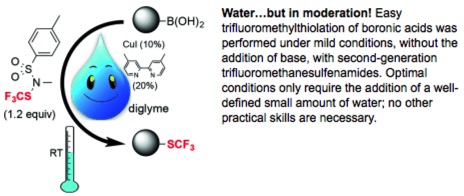
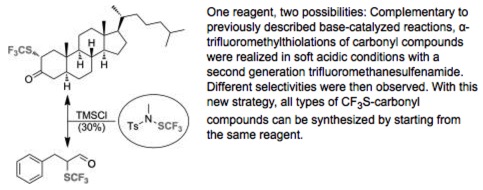
● Acid-Catalyzed Synthesis of α-Trifluoromethylthiolated Carbonyl Compounds
S. Alazet, E. Ismalaj, Q. Glenadel, D. Le Bars, T. Billard
Eur. J. Org. Chem. 2015, 4607-4610.
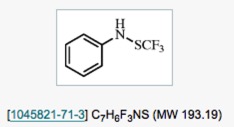
● 1,1,1-Trifluoro-N-phenylmethanesulfenamide
T. Billard
Encyclopedia of Reagents for Organic Synthesis, 2015.
● Le fluor en chimie organique : une montée en puissance (Fluorine in organic chemistry: gaining momentum)
T. Billard, E. Magnier, J.-P. Vors
Actualité Chimique 2015, 393-394, 56-61.

● Selective trifluoromethylthiolation of heteroaromatic sp2 C–H bonds with the 2nd generation of trifluoromethanesulfenamide reagent
S. Alazet, L. Zimmer, T. Billard
J. Fluorine Chem. 2015, 171, 78-81 (Special issue, on invitation).

● Preclinical evaluation of [18F]2FNQ1P as the first fluorinated serotonin 5-HT6 radioligand for PET imaging
G. Becker, J. Colomb, V. Sgambato-Faure, L. Tremblay, T. Billard, L. Zimmer
Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 495-502.
● Electrophilic Aromatic Trifluoromethylthiolation with the Second Generation of Trifluoromethanesulfenamide
S. Alazet, T. Billard
Synlett 2015, 26, 76-78 (On invitation).
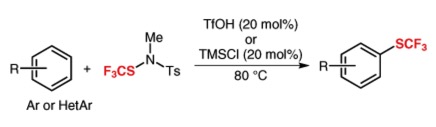
● Binding of the PET radiotracer [18F]BF227 does not reflect the presence of alpha-synuclein aggregates in transgenic mice
E. Levigoureux, S. Lancelot, C. Bouillot, F. Chauveau, M. Verdurand, J. Verchere, T. Billard, T. Baron, L. Zimmer
Curr. Alzheimer Res. 2014, 11, 955-960.
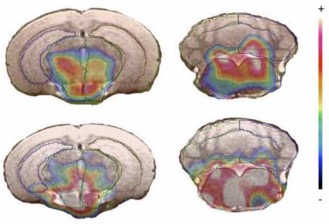
● (Re)Investigation of the reactivity of uranium hexafluoride toward several organic functions at room temperature
O. Roy, B. Marquet, J.-P. Alric, A. Jourdan, B. Morel, B. R. Langlois, T. Billard
J. Fluorine Chem. 2014, 167, 74-78 (Special issue, on invitation).
● 1,1,1-Trifluoro-N-methyl-N-phenyl-methanesulfenamide
T. Billard
Encyclopedia of Reagents for Organic Synthesis, 2014.
● A Postmortem Study to Compare Agonist and Antagonist 5-HT1A Receptor-binding Sites in Alzheimer's Disease
G. Becker, N. Streichenberger, T. Billard, A. Newman-Tancredi, L. Zimmer
CNS Neurosci. Ther. 2014, 20, 930-934.
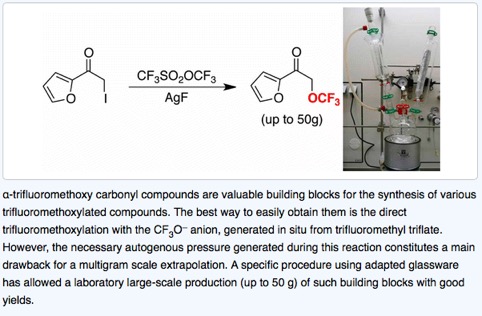
● Multigram Laboratory Scale Synthesis of α-Trifluoromethoxy Carbonyl Compounds
J. Barbion, S. Pazenok, J.-P. Vors, B. R. Langlois, T. Billard
Org. Process Res. Dev. 2014, 18, 1037-1040.
● Electrophilic Trifluoromethylthiolation of Carbonyl Compounds
S. Alazet, L. Zimmer, T. Billard
Chem. Eur. J. 2014, 20, 8589-8593.
● Molecular imaging of the serotonin 5-HT7 receptors: from autoradiography to positron emission tomography
L. Zimmer, T. Billard
Rev. Neurosci. 2014, 25, 357-365 (On invitation).

● Syntheses, Radiolabelings, and in Vitro Evaluations of Fluorinated PET Radioligands of 5-HT6 Serotoninergic Receptors
J. Colomb, G. Becker, S. Fieux, L. Zimmer, T. Billard
J. Med. Chem. 2014, 57, 3884-3890.
● Direct Trifluoromethylthiolation Reactions: The “Renaissance” of an Old Concept
F. Toulgoat, S. Alazet, T. Billard
Eur. J. Org. Chem. 2014, 2415-2428.
● Synthesis and pharmacological evaluation of a new series of radiolabeled ligands for 5-HT7 receptor PET neuroimaging
J. Colomb, G. Becker, E. Forcellini, S. Meyer, L. Buisson, L. Zimmer, T. Billard
Nucl. Med. Biol. 2014, 41, 330-337.
● PET Radiotracers for Molecular Imaging of Serotonin 5-HT1A Receptors
T. Billard, D. Le Bars, L. Zimmer
Curr. Med. Chem. 2014, 21, 70-81 (On invitation).

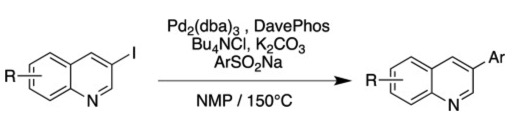
● Palladium-catalyzed desulfitative arylation of 3-haloquinolines with arylsulfinates
J. Colomb, T. Billard
Tetrahedron Lett. 2013, 54, 1471-1474.
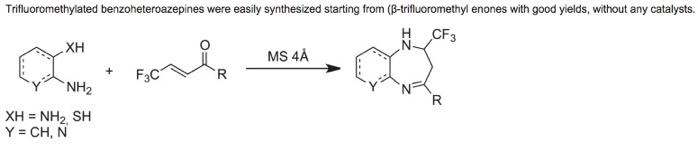
● Synthesis of trifluoromethylated 1,5-benzoheteroazepines
C. Christophe, B. R. Langlois, T. Billard
J. Fluorine Chem. 2013, 155, 118-123 (Special issue, on invitation).
● Direct electrophilic N-trifluoromethylthiolation of amines with trifluoromethanesulfenamide
S. Alazet, K. Ollivier, T. Billard
Beilstein J. Org. Chem. 2013, 9, 2354-2357 (Special issue, on invitation).
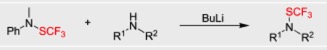
● Base-Catalyzed Electrophilic Trifluoromethylthiolation of Terminal Alkynes
S. Alazet, L. Zimmer, T. Billard
Angew. Chem. Int. Ed. 2013, 52, 10814-10817.
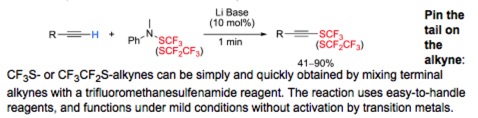
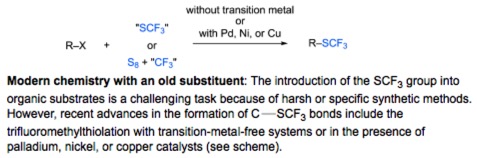
● Formation of C-SCF3 Bonds through Direct Trifluoromethylthiolation
A. Tlili, T. Billard
Angew. Chem. Int. Ed. 2013, 52, 6818-6819.
● Radiosynthesis and Preclinical Evaluation of 18F-F13714 as a Fluorinated 5-HT1A Receptor Agonist Radioligand for PET Neuroimaging
L. Lemoine, G. Becker, B. Vacher, T. Billard, S. Lancelot, A. Newman-Tancredi, L. Zimmer
J. Nucl. Med. 2012, 53, 969-976.
📖
● Electrophilic Trifluoromethanesulfanylation of Organometallic Species with Trifluoromethanesulfanamides
F. Baert, J. Colomb, T. Billard
Angew. Chem. Int. Ed. 2012, 51, 10382-10385.
📖
● Electrophilic trifluoromethanesulfanylation of indole derivatives
A. Ferry, T. Billard, E. Bacqué, B. R. Langlois
J. Fluorine Chem. 2012, 134, 160-163.
📖
● 1,4-Additions of electron-rich heterocycles onto [beta]-perfluoroalkyl enones
J. Leuger, G. Blond, T. Billard, G. Haufe, B. R. Langlois
J. Fluorine Chem. 2011, 132, 799-803 (Special issue, on invitation).
📖
● Comparison of 4 Radiolabeled Antagonists for Serotonin 5-HT7 Receptor Neuroimaging: Toward the First PET Radiotracer
L. Lemoine, J. Andries, D. Le Bars, T. Billard, L. Zimmer
J. Nucl. Med. 2011, 52, 1811-1818.
📖
● Synthesis and biological evaluation of potential 5-HT7 receptor PET radiotracers
J. Andries, L. Lemoine, D. Le Bars, L. Zimmer, T. Billard
Eur. J. Med. Chem. 2011, 46, 3455-3461.
📖
● Synthesis of β-Trifluoromethylated Δ1-Pyrrolines
O. Marrec, C. Christophe, T. Billard, B. Langlois, J.-P. Vors, S. Pazenok
Adv. Synth. Catal. 2010, 352, 2825-2830.
📖
● A New and Direct Trifluoromethoxylation of Aliphatic Substrates with 2,4-Dinitro(trifluoromethoxy)benzene
O. Marrec, T. Billard, J.-P. Vors, S. Pazenok, B. R. Langlois
Adv. Synth. Catal. 2010, 352, 2831-2837.
📖
● A deeper insight into direct trifluoromethoxylation with trifluoromethyl triflate
O. Marrec, T. Billard, J.-P. Vors, S. Pazenok, B. R. Langlois
J. Fluorine Chem. 2010, 131, 200-207.
📖
● Looking for a 5-HT7 radiotracer for positron emission tomography
J. Andriès, L. Lemoine, A. Mouchel-Blaisot, S. Tang, M. Verdurand, D. Le Bars, L. Zimmer, T. Billard
Bioorg. Med. Chem. Lett. 2010, 20, 3730-3733.
📖
● Synthesis of beta -Trifluoromethylated enones: an unexpected reactivity of trifluoromethylated weinreb enamides towards organolithium species
O. Marrec, J. Borrini, T. Billard, B. R. Langlois
Synlett 2009, 1241-1244.
📖
● Trifluoromethanesulfanylamides as Easy-to-Handle Equivalents of the Trifluoromethanesulfanyl Cation (CF3S+): Reaction with Alkenes and Alkynes
A. Ferry, T. Billard, B. R. Langlois, E. Bacqué
Angew. Chem. Int. Ed. 2009, 48, 8551-8555.
📖
● 2,2'-Bipyridine-3,3'-dicarboxylic carbohydrate esters and amides. Synthesis and preliminary evaluation as ligands in Cu(II)-catalysed enantioselective electrophilic fluorination
A. Assalit, T. Billard, S. Chambert, B. R. Langlois, Y. Queneau, D. Coe
Tetrahedron Asymmetry 2009, 20, 593-601.
📖
● Synthesis of trifluoromethanesulfinamidines and -sulfanylamides
A. Ferry, T. Billard, B. R. Langlois, E. Bacque
J. Org. Chem. 2008, 73, 9362-9365.
📖
● Use of cyclic alpha ,beta -unsaturated trifluoromethyl sulfones in Diels-Alder reactions and Michael additions
T. Billard, B. R. Langlois, M. Essers, G. Haufe
Collect. Czech. Chem. Commun. 2008, 73, 1814-1824 (Special issue, on invitation).
📖
● Synthesis and biological evaluation in rat and cat of [18F]12ST05 as a potential 5-HT6 PET radioligand
S. Tang, M. Verdurand, B. Joseph, L. Lemoine, A. Daoust, T. Billard, G. Fournet, D. Le Bars, L. Zimmer
Nucl. Med. Biol. 2007, 34, 995-1002.
📖
● A new preparation of trifluoromethanesulfinate salts
B. R. Langlois, T. Billard, J.-C. Mulatier, C. Yezeguelian
J. Fluorine Chem. 2007, 128, 851-856.
📖
● How to reach stereogenic trifluoromethylated carbon? En route to the "grail" of the asymmetric trifluoromethylation reaction
T. Billard, B. R. Langlois
Eur. J. Org. Chem. 2007, 891-897.
📖
● Synthetic Applications of alpha -Fluoroalkylated Enones. 1. Use as Dienophiles in Diels-Alder Cycloadditions
J. Leuger, G. Blond, R. Froehlich, T. Billard, G. Haufe, B. R. Langlois
J. Org. Chem. 2006, 71, 2735-2739.
📖
● Synthetic applications of beta -fluoroalkylated alpha ,beta -unsaturated carbonyl compounds
T. Billard
Chem. Eur. J. 2006, 12, 974-979.
📖
● Towards enantioselective nucleophilic trifluoromethylation
S. Roussel, T. Billard, B. R. Langlois, L. Saint-James
Chem. Eur. J. 2005, 11, 939-944.
📖
● Nucleophilic trifluoromethylation. Some recent reagents and their stereoselective aspects
B. R. Langlois, T. Billard, S. Roussel
J. Fluorine Chem. 2005, 126, 173-179.
📖
● Towards the syntheses of alpha -trifluoromethylated oxygenated heterocycles and their precursors
S. Harthong, T. Billard, B. R. Langlois
Synthesis 2005, 2253-2263.
📖
● Synthesis of alpha -Trifluoromethylated nitrogen bicycles
A. Ferry, T. Billard, B. R. Langlois
Synlett 2005, 1027-1029.
📖
● A concise synthesis of trifluoromethylated cyclohexenones: A one-pot, five-step domino reaction
C. Christophe, T. Billard, B. R. Langlois
Eur. J. Org. Chem. 2005, 3745-3748.
📖
● From fluoral to heterocycles: a survey of polyfluorinated iminium chemistry
T. Billard, S. Gille, A. Ferry, A. Barthelemy, C. Christophe, B. R. Langlois
J. Fluorine Chem. 2005, 126, 189-196.
📖
● Electrophilic aromatic fluorination with fluorine: meta-Directed fluorination of anilines
J. P. Alric, B. Marquet, T. Billard, B. R. Langlois
J. Fluorine Chem. 2005, 126, 661-667.
📖
● Trifluoromethanesulfinamide from ephedrine: A more efficient trifluoromethylating reagent
S. Roussel, T. Billard, B. R. Langlois, L. Saint-Jalmes
Synlett 2004, 2119-2122.
● Fluoride-assisted trifluoromethylation of aromatic thiones with (trifluoromethyl)trimethylsilane
S. Large-Radix, T. Billard, B. R. Langlois
J. Fluorine Chem. 2003, 124, 147-149.
● Some recent results in nucleophilic trifluoromethylation and introduction of fluorinated moieties
B. R. Langlois, T. Billard
Synthesis 2003, 185-194.
● Trifluoroacetamides from amino alcohols as nucleophilic trifluoromethylating reagents
J. Joubert, S. Roussel, C. Christophe, T. Billard, B. R. Langlois, T. Vidal
Angew. Chem. Int. Ed. 2003, 42, 3133-3136.
● Trifluoroacetic acid derivatives as nucleophilic trifluoromethylating reagents
L. Jablonski, J. Joubert, T. Billard, B. R. Langlois
Synlett 2003, 230-232.
● Trifluoroacetophenone as nucleophilic trifluoromethylating reagent
L. Jablonski, T. Billard, B. R. Langlois
Tetrahedron Lett. 2003, 44, 1055-1057.
● Trifluoromethanesulfinic acid derivatives as nucleophilic trifluoromethylating reagents
D. Inschauspe, J.-B. Sortais, T. Billard, B. R. Langlois
Synlett 2003, 233-235.
● Synthesis of alpha -Trifluoromethylated Nitrogen Heterocycles
S. Gille, A. Ferry, T. Billard, B. R. Langlois
J. Org. Chem. 2003, 68, 8932-8935.
● Trifluoromethylation reactions with potassium trifluoromethanesulfinate under electrochemical oxidation
J.-B. Tommasino, A. Brondex, M. Medebielle, M. Thomalla, B. R. Langlois, T. Billard
Synlett 2002, 1697-1699.
● Reactivity of stable trifluoroacetaldehyde hemiaminals. Part 3. Generation and use of an equivalent of difluoroacetamide or difluoroacetate anions
G. Blond, T. Billard, B. R. Langlois
Chem. Eur. J. 2002, 8, 2917-2922.
● Reactivity of Stable Trifluoroacetaldehyde Hemiaminals. 2. Generation and Synthetic Potentialities of Fluorinated Iminiums
T. Billard, B. R. Langlois
J. Org. Chem. 2002, 67, 997-1000.
● Synthesis of 4-fluorophenols from 4-tert-butylphenols and fluoride sources under oxidative conditions
A. Bienvenu, A. Barthelemy, S. Boichut, B. Marquet, T. Billard, B. R. Langlois
Collect. Czech. Chem. Commun. 2002, 67, 1467-1478 (Special issue, on invitation).
● Telomerization of vinylidene fluoride with alkyl (or aryl) trifluoromethanethiosulfonates
B. Ameduri, T. Billard, B. Langlois
J. Polym. Sci., Part A: Polym. Chem. 2002, 40, 4538-4549.
● Reactivity of Stable Trifluoroacetaldehyde Hemiaminals. 1. An Unexpected Reaction with Enolizable Carbonyl Compounds
G. Blond, T. Billard, B. R. Langlois
J. Org. Chem. 2001, 66, 4826-4830.
● New stable reagents for the nucleophilic trifluoromethylation. Part 4. Trifluoromethylation of disulfides and diselenides with hemiaminals of trifluoroacetaldehyde
G. Blond, T. Billard, B. R. Langlois
Tetrahedron Lett. 2001, 42, 2473-2475.
● Tetrakis(dimethylamino)ethylene (TDAE) mediated addition of difluoromethyl anions to heteroaryl thiocyanates. A new simple access to heteroaryl-SCF2R derivatives
T. Billard, B. R. Langlois, M. Medebielle
Tetrahedron Lett. 2001, 42, 3463-3465.
● New stable reagents for nucleophilic trifluoromethylation, 3. Trifluoromethylation of nonenolizable carbonyl compounds with a stable piperazino hemiaminal of trifluoroacetaldehyde
T. Billard, B. R. Langlois, G. Blond
Eur. J. Org. Chem. 2001, 1467-1471.
● Synthetic uses of thioesters of trifluoromethylated acids. Part 2. Reactions with alkenes
T. Billard, N. Roques, B. R. Langlois
Tetrahedron Lett. 2000, 41, 3069-3072.
● New stable reagents for nucleophilic trifluoromethylation. Part 2: Trifluoromethylation with silylated hemiaminals of trifluoroacetaldehyde
T. Billard, B. R. Langlois, G. Blond
Tetrahedron Lett. 2000, 41, 8777-8780.
● New Stable Reagents for the Nucleophilic Trifluoromethylation. 1. Trifluoromethylation of Carbonyl Compounds with N-Formylmorpholine Derivatives
T. Billard, S. Bruns, B. R. Langlois
Org. Lett. 2000, 2, 2101-2103.
● Electrophilic trifluoromethylation of vinyl sulfides
B. R. Langlois, T. Billard, S. Guerin, S. Large, N. Roidot-Perol
Phosphorus, Sulfur Silicon Relat. Elem. 1999, 153-154, 323-324.
● Synthetic Uses of Thio- and Selenoesters of Trifluoromethylated Acids. 1. Preparation of Trifluoromethyl Sulfides and Selenides
T. Billard, N. Roques, B. R. Langlois
J. Org. Chem. 1999, 64, 3813-3820.
● Addition of trifluoromethanethio- and trifluoromethaneselenosulfonates to olefins. Synthesis of vinyl triflones
T. Billard, B. R. Langlois
Tetrahedron 1999, 55, 8065-8074.
● A new equivalent of the CF3S(O)+ cation. Synthesis of trifluoromethanesulfinates and trifluoromethanesulfinamides
T. Billard, A. Greiner, B. R. Langlois
Tetrahedron 1999, 55, 7243-7250.
● Synthesis of trifluoromethyl selenides
T. Billard, B. R. Langlois, S. Large
Phosphorus, Sulfur Silicon Relat. Elem. 1998, 136,137&138, 521-524.
● Preparation of trifluoromethyl sulfides or selenides from trifluoromethyl trimethylsilane and thiocyanates or selenocyanates
T. Billard, S. Large, B. R. Langlois
Tetrahedron Lett. 1997, 38, 65-68.
● A new synthesis of thioesters and selenoesters of triflic acid under oxidative conditions
T. Billard, B. R. Langlois
J. Fluorine Chem. 1997, 84, 63-64.
● A New Route to Thio- and Selenosulfonates from Disulfides and Diselenides. Application to the Synthesis of New Thio- and Selenoesters of Triflic Acid
T. Billard, B. R. Langlois, S. Large, D. Anker, N. Roidot, P. Roure
J. Org. Chem. 1996, 61, 7545-7550.
● A new simple access to trifluoromethyl thioethers or selenoethers from trifluoromethyl trimethylsilane and disulfides or diselenides
T. Billard, B. R. Langlois
Tetrahedron Lett. 1996, 37, 6865-6868.